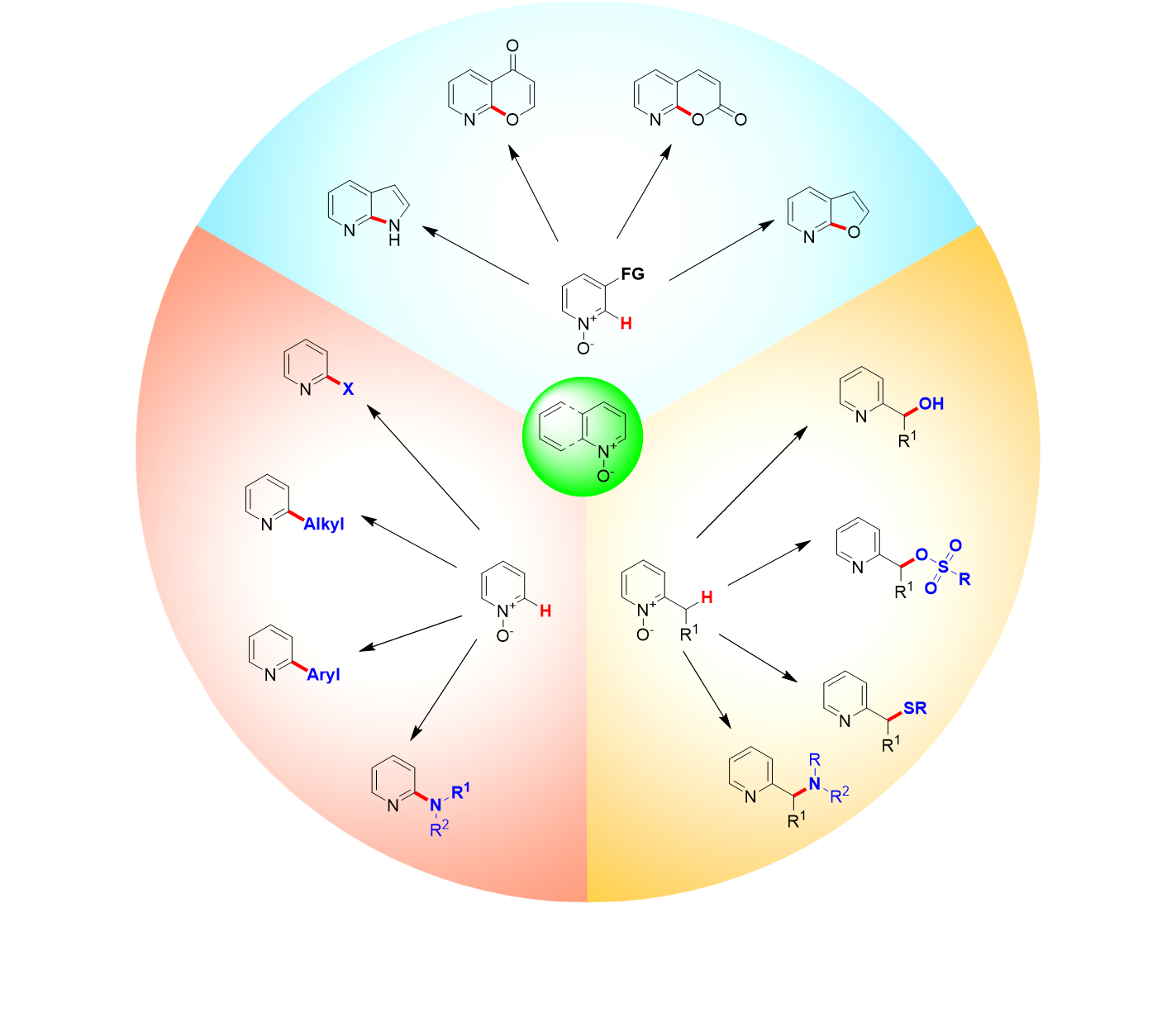
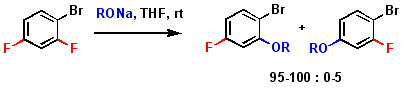Recent advances in the synthesis of C2-functionalized pyridines and quinolines using N-oxide chemistry. Wang D., Désaubry L., Li G., Huang M., Zheng S. Adv. Synth. Catal. 363: 2-39 (2020).
Unexpected inversion of configuration during the carbamoylation of 1-azaflavaglines. Abou-Hamdan H., Désaubry L. Synlett, 31: 2023-2026 (2020).
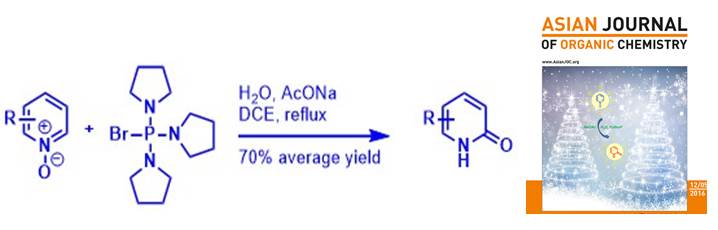
 Accessing 1,8-naphthyridones by metal-free regioselective amination of pyridine N-oxides. Zhao L., Hao L., Fu Y., Cheng Y., Pan G., Désaubry L., Yu P., Dong Wang D. Adv. Synth. Catal. 362: 3841-3845 (2020).
Accessing 1,8-naphthyridones by metal-free regioselective amination of pyridine N-oxides. Zhao L., Hao L., Fu Y., Cheng Y., Pan G., Désaubry L., Yu P., Dong Wang D. Adv. Synth. Catal. 362: 3841-3845 (2020).
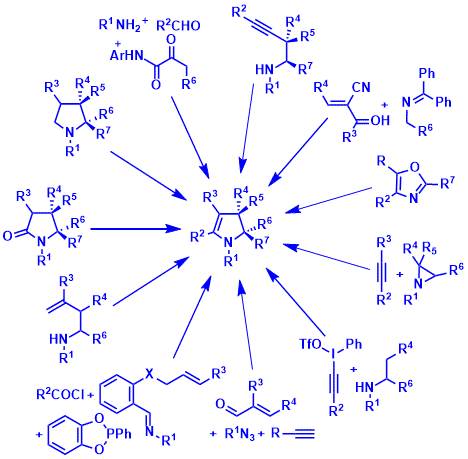
 Recent advances in the synthesis of 2,3-dihydropyrroles. Wang D., Fan Y., Yu P., Désaubry L. Chem. Commun. 56: 5584-5592 (2020).
Recent advances in the synthesis of 2,3-dihydropyrroles. Wang D., Fan Y., Yu P., Désaubry L. Chem. Commun. 56: 5584-5592 (2020).
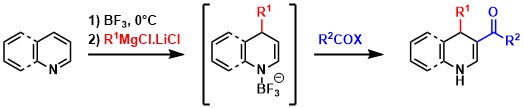
 A one-pot selective saturation and functionalization of heteroaromatics leading to dihydropyridines and dihydroquinolines. Wang D, Jiang Y, Dong L, Li G, Sun B, Désaubry L, Yu P. J. Org. Chem. 85: 5027-5037 (2020).
A one-pot selective saturation and functionalization of heteroaromatics leading to dihydropyridines and dihydroquinolines. Wang D, Jiang Y, Dong L, Li G, Sun B, Désaubry L, Yu P. J. Org. Chem. 85: 5027-5037 (2020).
 Stereoselective four-component synthesis of functionalized 2,3- dihydro-4-nitropyrroles. Wang D., Ma X., Feng H., Yu P., Désaubry L. Front. Chem. 7: 810 (2019).
Stereoselective four-component synthesis of functionalized 2,3- dihydro-4-nitropyrroles. Wang D., Ma X., Feng H., Yu P., Désaubry L. Front. Chem. 7: 810 (2019).
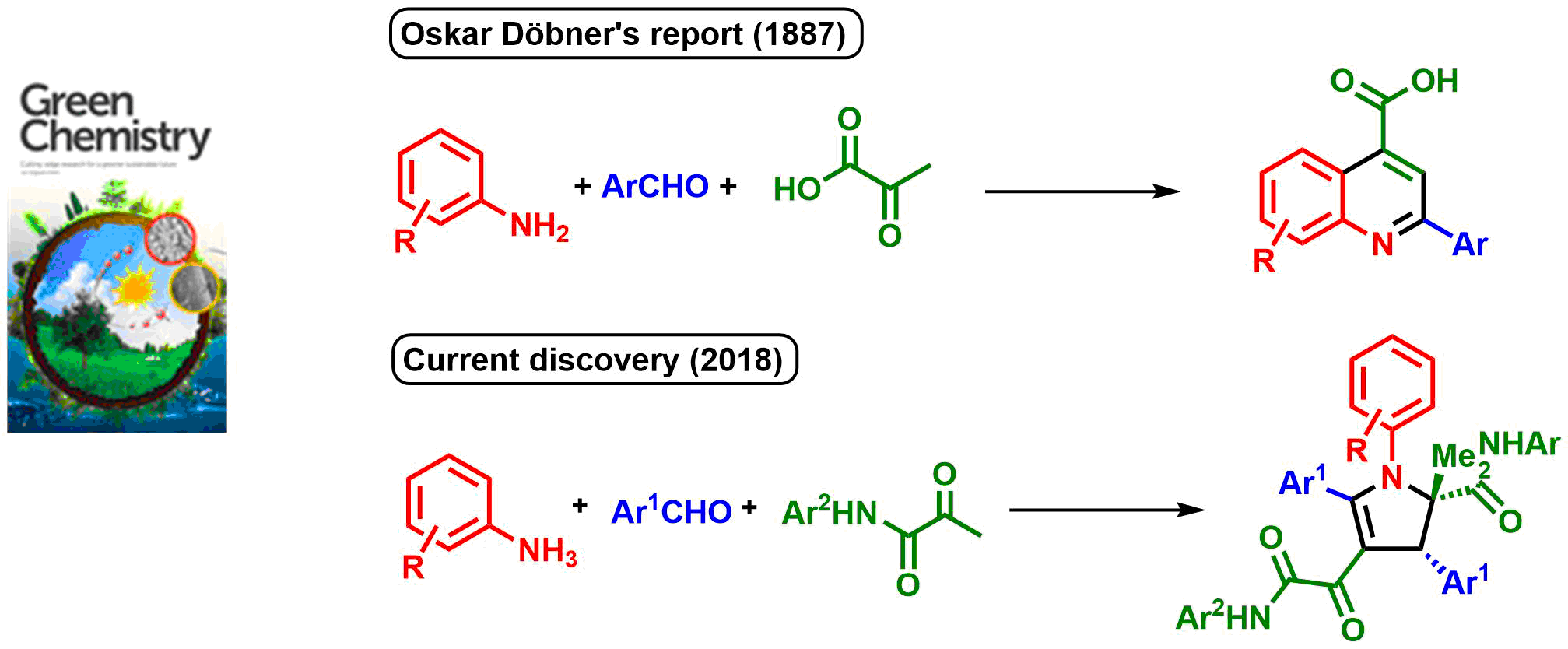
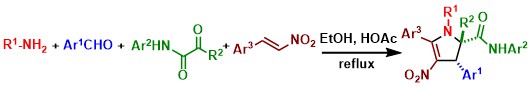 Catalyst-free three-component synthesis of highly functionalized 2,3-dihydropyrroles. Wang D., Li L., Feng H., Sun H., Almeida-Veloso F., Charavin M., Yu P., Désaubry L. Green Chem. 20: 2775-2780 (2018).
Catalyst-free three-component synthesis of highly functionalized 2,3-dihydropyrroles. Wang D., Li L., Feng H., Sun H., Almeida-Veloso F., Charavin M., Yu P., Désaubry L. Green Chem. 20: 2775-2780 (2018).
Scalable 9-Step Synthesis of the Splicing Modulator NVS-SM2. Abou-Hamdan H., Désaubry L. J. Org. Chem. 83: 2954-2958 (2018).
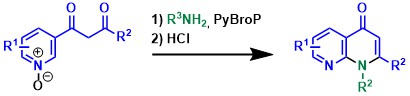
A general and efficient synthesis of 2-pyridones, 2-quinolinones and 1-isoquinolinones from azine-N-oxides. Wang D., Zhao J., Wang Y., Hu J., Li L., Miao L., Feng H., Désaubry L;, Yu P. Asian J. Org. Chem. 5: 1442–1446 (2016).
Synthesis of hydroxybenzofurans by condensation of quinones with benzoylacetone: revised structure of the adducts. Hammoud H., Zhao Q., Désaubry L. Tetrahedron Lett. 57: 4044–4045 (2016).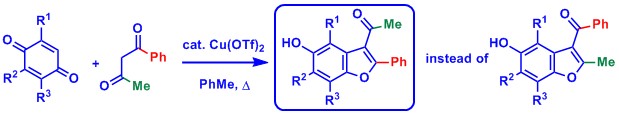
Recent advances in the synthesis of flavaglines, a family of potent bioactive natural compounds coming from traditional Chinese medicine. Zhao Q., Abou-Hamdan H., Désaubry L. Eur. J. Org. Chem. 1442–1446 (2016).
Bioisosteric modification of flavaglines. Zhao Q., Tijeras-Raballand A., de Gramont A., Raymond E., Désaubry L. Tetrahedron Lett. 57: 2943–2944 (2016).
Exploratory studies toward a synthesis of flavaglines. A novel access to a highly substituted cyclopentenone intermediate. Basmadjian C., Zhao Q., Désaubry L. Tetrahedron lett. 56: 727–730 (2015).
Novel carbocationic rearrangements of 1-styryl propargyl alcohols. Basmadjian C., Zhang F., Désaubry L. Beilstein. J. Org. Chem. 11: 1017–1022 (2015).
Unprecedented Elimination of Conjugated Phenylthioether Groups by Low-Valent Titanocene. Ribeiro N., Fetzer L., Streiff S., Désaubry L. Synlett 2928-2930 (2010).![]()
Regioselectivity of fluorine substitution by alkoxides on unsymmetrical difluoroarenes. Dirr R., Antheaume C, Désaubry L. Tetrahedron Lett. 49: 4588-4590 (2008).
Tandem Diels–Alder-manganese dioxide mediated oxidation reaction. A short route to marcanines. Mekideche S., Désaubry L. Tetrahedron Lett. 49: 5268-5270 (2008). Reinforcing effect of bi- and tri-cyclopolyprenols on “primitive” membranes made of polyprenyl phosphates. Ribeiro N.,Streiff S., Heissler D., Elhabiri M., Albrecht-Gary A-M., Atsumi M., Gotoh M., Désaubry L., Nakatani Y, and Ourisson G. Tetrahedron 63: 3395-3407 (2007).
Reinforcing effect of bi- and tri-cyclopolyprenols on “primitive” membranes made of polyprenyl phosphates. Ribeiro N.,Streiff S., Heissler D., Elhabiri M., Albrecht-Gary A-M., Atsumi M., Gotoh M., Désaubry L., Nakatani Y, and Ourisson G. Tetrahedron 63: 3395-3407 (2007).
Looking forward: a glance into the future of organic chemistry. Compain P. et al. New J. Chem. 30: 823-831 (2006).
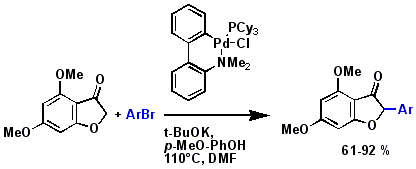
Palladium catalysed arylation of 6,8-dimethoxybenzofuranone. Sani Souna Sido A., Boulenger L., Désaubry L. Tetrahedron Lett. 46: 8017-8018 (2005).
Concise synthesis and voltammetric studies of dielsiquinone, a cytotoxic azaanthraquinone. Brisach-Wittmeyer A., Sani Souna Sido A., Guilini P., Désaubry L. Bioorg. Med. Chem. Lett. 15, 3609-10 (2005). Synthesis of allylsilanes by reductive lithiation of thioethers. Streiff S., Ribeiro N., Désaubry L. J. Org. Chem. 69: 7592-7598 (2004).
Synthesis of allylsilanes by reductive lithiation of thioethers. Streiff S., Ribeiro N., Désaubry L. J. Org. Chem. 69: 7592-7598 (2004).
A Novel Type of Membranes based on Cholesteryl Phosphate. Sedaghat S., Désaubry L., Streiff S., Ribeiro N., Michels B., Nakatani Y., Ourisson G. Chem. Biodiversity 1: 124-128 (2004).
Unexpected cleavage of tetrahydrofuran by catalytic reductive lithiation. Streiff S., Ribeiro N., Désaubry L. Chem. Commun. 346-347 (2004).
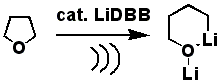 Toward higher polyprenols under “prebiotic” conditions. Désaubry L., Nakatani Y., Ourisson G. Tetrahedron Lett. 44: 6959-6961 (2003).
Toward higher polyprenols under “prebiotic” conditions. Désaubry L., Nakatani Y., Ourisson G. Tetrahedron Lett. 44: 6959-6961 (2003).
One-pot synthesis of deoxyadenosine 3′-thiophosphate. Szczepanik M. B., Désaubry L., Johnson R.A. Tetrahedron Lett. 7455-7458 (1998). Conjugation of nucleoside triphosphates to an amino linker. Désaubry L., Johnson, R.A. Bioorg. Med. Chem. Lett. 7: 123-126 (1997).
Conjugation of nucleoside triphosphates to an amino linker. Désaubry L., Johnson, R.A. Bioorg. Med. Chem. Lett. 7: 123-126 (1997).
Regio-controlled substitution of 9-substituted purines. Désaubry L., Wermuth C.G., Bourguignon, J.J. Tetrahedron Lett. 36: 7875-7876 (1995). Synthesis of 2′, 5′-Dideoxy-adenosine-3′-monophosphate derivatives as allosteric inhibitors of adenylyl cyclase. Désaubry L., Shoshani I., Johnson R.A. Nucleosides and Nucleotides. 14: 1453-1460 (1995).
Synthesis of 2′, 5′-Dideoxy-adenosine-3′-monophosphate derivatives as allosteric inhibitors of adenylyl cyclase. Désaubry L., Shoshani I., Johnson R.A. Nucleosides and Nucleotides. 14: 1453-1460 (1995).
Synthesis of 2′-Deoxy- and 2′-5′-Dideoxy-Adenosine-3′-Di- and 3′-Triphosphate. Désaubry L., Shoshani I., Johnson R.A. Tetrahedron Lett. 36: 995-996 (1995).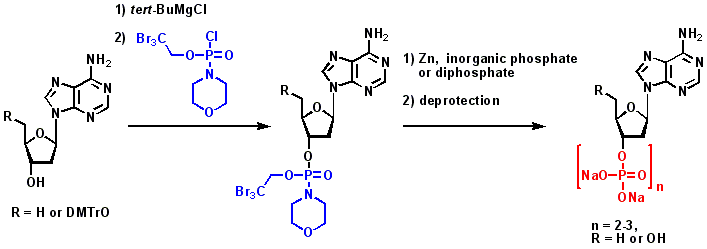
Synthesis of conformationally constrained analogue of BW A78U, an anticonvulsant adenine derivative. Désaubry L.,Wermuth C.G., Bourguignon J.J. Tetrahedron Lett. 36: 4249-4252 (1995).